The Lewis structure of CH3OCH3 is dimethyl ether the simplest ether in existence. So the skeletal formula for dimethyl ether looks like this.

Draw A Lewis Structure Of Formaldehyde Lewis College Life Hacks Study Tips
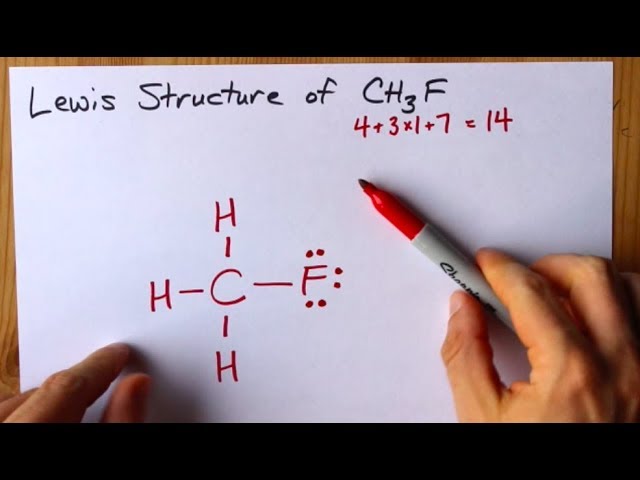
CH 3 F fluoromethane has one carbon atom three hydrogen atoms and one fluorine atom.

. Problem 84 Hard Difficulty. The first step is to sketch the Lewis structure of the CHF3 molecule to add valence electrons around the carbon atom. While drawing the Lewis structure for CH3OH you will notice that the Carbon atom will have three bonds with three hydrogen atoms and one bond with the Hydroxyl Group.
Carbons valency is 4 4 single bonds2double bonds Hydrogens is 1 1 single bond Oxygens is 2 2 single bonds1 double bond So for CH3OCH3 each Carbon atom bonds to three Hydrogen atoms and the central Oxygen atom. For the CH3OCH3 structure use the periodic table to find the total number of valence electrons for the CH3OCH3 molecule. Draw a lewis structure of the conjugate acid of CH3OCH3 Offered Price.
Since we know valence electrons we can find the total electron pairs of CH3CH2CH3 molecule. Individual results may vary. And well put the last 4 on the center Oxygen to complete its octet.
The structure of the organic compound contains a total of 20 valence electrons. 10272015 1245 PM Due on. When we are drawing a CH3OCH3 molecule we must remember that it consists of the ether R-O-R here RR group.
CH3CH2CH3 has 10 total electron pairs. Get Your Custom Essay on. 11262015 Question 00124900 Subject Chemistry Topic General Chemistry Tutorials.
The second step is to add valence electrons to the one fluorine and three hydrogen atoms and the final step is to combine the step1 and. CH3OCH3 Lewis Structure CH 3 OCH 3 dimethyl ether has two carbon atoms six hydrogen atoms and one oxygen atom. Dont use plagiarized sources.
Hydrogen always goes on the outside of Lewis structures. Chegg survey fielded between April 23-April 25 2021 among customers who used Chegg Study and Chegg Study Pack in Q1 2020 and Q2 2021. 1 Chemical Foundations 2 Atoms Molecules And Ions 3 Stoichiometry 4 Types Of Chemical Reactions And Solution Stoichiometry 5 Gases 6 Thermochemistry 7 Atomic Structure And Periodicity 8 Bonding.
400 Posted By. We can see that oxygen has acted as the central atom in. In the Lewis structure the central oxygen atom is bonded to two carbon atoms which have little or no electronegativity.
And on the oxygen atom there are two lone pairs. Draw the Lewis structure for each organic compound from its condensed structural formula. Well put 2 between the atoms to form chemical bonds so weve used 16.
For the CH3OCH3 Lewis structure we have a total of 20 valence electrons. Draw the Lewis structure of CH3F. And then Carbon is less electronegative than Fluorine so lets put the Carbon in the center and the Hydrogens on the outside there and the Fluorine on the top.
General Concepts 9. We need to divide total valence electrons by 2. Draw a lewis structure of the conjugate acid of CH3OCH3.
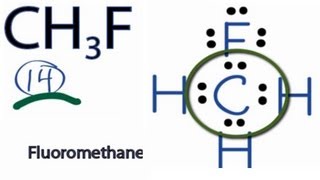
First determine the valency of each atom involved. In the lewis structure of CH 3 F there are four single bonds around the carbon atom with three hydrogen atoms and one fluorine atom attached to it and on the fluorine atom there are three lone pairs. For the molecule CH3OCH3 dimethyl ethera Draw the Lewis structure of the moleculeb Identify where the lone pair electrons are locatedc Identify the n.
The Lewis structure of CH3OCH3 is dimethyl ether the simplest ether in existence. The second step is to add valence electrons to the three fluorine and one hydrogen atoms and the final step is to combine the step1 and. What is the Lewis structure of CH3OCH3.
H O N S Р F Br CI 1 X More Submit Request Answer. So weve used all 20 valence electrons. In the Lewis structure the central oxygen atom is bonded to two carbon atoms which have little or no electronegativity.
This is the Lewis structure for CH3F. The six remaining hydrogen atoms in the molecule are evenly. For CH3F we have a total of 14 valence electrons.
How do you determine the Lewis structure of CH3OCH3. Carbon and Hydrogen follow octet rule. Draw the molecule by placing atoms on the grid and connecting them with bonds.
Draw the molecule by placing atoms on the grid and connecting them with bonds. As the Carbon has four valence electrons that form the bonds with other atoms it shows sp3 hybridization. Include all lone pairs of electrons and all hydrogen atoms.
The first step is to sketch the Lewis structure of the CH3F molecule to add valence electron around the carbon atom. Draw the Lewis structure of CH3F. The structure of the organic compound contains a total of 20 valence electrons.
Once we know how many valence electr. Center atom should be Carbon There are no lone pairs. Respondent base n745 among approximately 144000 invites.
The Lewis structure of CH3OCH3 is dimethyl ether the simplest ether in existence. Each of the Carbons has 8. Here you have the CH3CH2CH3 Lewis Structure.
In the lewis structure of CH 3 OCH 3 there are two single bonds around the oxygen atom with two carbon atoms attached to it and each carbon is attached with three hydrogen atoms. Survey respondents up to 500000 respondents total were entered into a drawing to win 1 of 10 500 e-gift cards.

How To Draw Ch3f Lewis Structure Science Education And Tutorials

Ch3f Lewis Structure How To Draw The Lewis Structure For Ch3f Fluormethane Youtube

Ch3f Lewis Structure Molecular Geometry Bond Angle Polarity Electrons
How To Draw The Lewis Structure Of Ch3f Fluoromethane Youtube

How To Draw Ch3f Lewis Structure Science Education And Tutorials
Ch3f Lewis Structure How To Draw The Lewis Structure For Ch3f Fluormethane Youtube

How To Draw Ch3f Lewis Structure Science Education And Tutorials

Ch3f Lewis Structure Molecular Geometry Hybridization And Polarity Techiescientist
0 comments
Post a Comment